number of electrons in br|formula to find electrons : Baguio Scientist Chadwick discovered neutrons in 1932. It is located in the nucleus at the center of the atom. The neutron is a charge . Tingnan ang higit pa How to convert Philippine pesos to Japanese yen. 1 Input your amount. Simply type in the box how much you want to convert. 2 Choose your currencies. Click on the dropdown to select PHP in the first dropdown as the currency that you want to convert and JPY in the second drop down as the currency you want to convert to.
number of electrons in br,When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. The ionic properties of the elements depend on the exchange of electrons. In an atomic ion only the number of electrons changes but the number of protons and neutrons does not change. . Tingnan ang higit pa
An atom is the smallest particle of an element that has no independent existence but is directly involved in chemical . Tingnan ang higit pa
Protons are the permanent core particles of an atom. It resides in the center or nucleus of the atom. When a hydrogen atom removes an electron from its orbit, the positively charged particle that remains is called a . Tingnan ang higit paScientist Chadwick discovered neutrons in 1932. It is located in the nucleus at the center of the atom. The neutron is a charge . Tingnan ang higit paElectrons are the permanent core particles of an atom. It resides in a specific orbit of the atom and revolves around the nucleus. The properties of the elements and their compounds depend on the electron configuration. . Tingnan ang higit panumber of electrons in br Answer link. The "Br"^ (-)" ion has 36 electrons. The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine .Atomic numberThe number of protons in an atom. Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at .
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number .

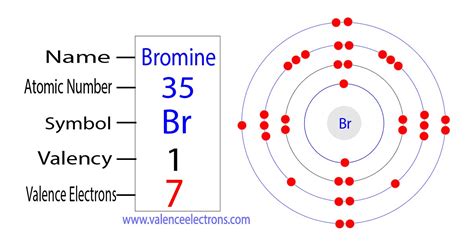
How to find Protons & Electrons for the Bromide ion (Br-) Wayne Breslyn. 95 Likes. 2020 Sep 12. In this video we’ll use the Periodic table and a few simple rules to . 135. 16K views 3 years ago. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Bromine .Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is .Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine .
Bromine Electron Configuration: Bromine (Br) is a chemical element. The atomic number of bromine is 35. It is the fuming red-brown liquid at room temperature and the third-lightest halogen. It evaporates .

Solution. Verified by Toppr. The atomic number of bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a 1 − bromide ion, it would have to gain an additional electron. So, the no. of electrons in B r − = 36.
Atomic Number of Bromine. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons .
number of electrons in br formula to find electrons Correctc. 28. Bromine has 28 core electrons. no its not 35 that includes valence electrons. The core electrons are those in the filled energy level. Therefore it is C. It has the 1s, 2s, 2p, 3s, 3p, 3d filled making twenty eight core electrons. the 4s, and partial 4p are valence. Dan,Atomic Number – Protons, Electrons and Neutrons in Bromine. Bromine is a chemical element with atomic number 35 which means there are 35 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e .The atomic number of Bromine Br is 35. The electronic configuration of Bromine Br can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5; The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a total of seven valence electrons. Therefore, the valence .formula to find electrons - We know the atomic number of bromine. - The atomic number of bromine is 35, which means the bromine element contains 35 electrons and 35 protons. - But in the question it is given that to find the number of protons and electrons for bromide anion. - Bromide anion means bromine accepts one electron so the number electrons in .
To find out the atomic number of bromine, we can use the periodic table. With the help of the periodic table, we can easily see that the atomic number of bromine is 35. As its atomic number is 35, it has 35 protons, and for neutral bromine, the number of protons is always equal to the number of electrons i.e. has 35 electrons in its nucleus.
Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. Bromine Electron Configuration: Bromine (Br) is a chemical element. The atomic number of bromine is 35. It is the fuming red-brown liquid at room temperature and the third-lightest halogen. It evaporates readily and forms a colored gas. . Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5.Bromine is a chemical element; it has symbol Br and atomic number 35. . (2.55), the carbon atom in a C–Br bond is electron-deficient and thus electrophilic. The reactivity of organobromine compounds resembles but is intermediate between the reactivity of organochlorine and organoiodine compounds. For many applications, organobromides .Atomic Structure: An atom is made up of three subatomic particles: protons, neutrons, and electrons. Inside the nucleus of the atom are the positively charged protons and the neutrally charge neutrons. These two subatomic particles have a relative mass of 1. Surrounding the nucleus of an atom are the negatively charged electrons which are .The other halogen molecules (F 2, Br 2, I 2, and At 2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. This allows each halogen atom to have a noble gas electron configuration. . H 3 CCH 3: all atoms have the correct number of electrons HCCH: form a triple bond between .For Bromine the atomic number is 35. so number of protons = 35. In a neutral atom the number of protons = the number of electrons. 35 protons = 35 electrons. The atomic mass is the sum of the number of protons and the number of neutrons (as electrons weigh almost nothing) so. Protons + neutrons = Atomic mass atomic mass of br is 80Total number of electrons of the valance shells of Br 2. There is only one element in bromine molecule. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of bromine atoms.
Determine the number of unpaired electrons on each of the following, using orbital diagrams to prove your answer: (a) Ni (b) Fe^ {3+} (c) S (d) Al^ {3+} (e) Br^-. Write the orbital diagram for sulfur and determine the number of unpaired electrons. Predict the number of unpaired electrons in the [Cr (en)3]2+ion.5. For ions with the same number of protons, the one with more electrons will be larger due to increased electron-electron repulsion. Therefore, Se2- (with 34 protons) will be larger than Br- (with 35 protons). Answer 6. For ions with the same number of protons and electrons, the one with more electron shells will be larger due to increased .
Bromine is a member of the halogen family of elements. Its companions include fluorine, chlorine, and iodine. Like the other halogens, bromine has seven electrons in its outer shell and is very reactive. You will find bromine in many salt compounds with alkali metals. Sodium bromide is a compound found in seawater.How many electrons are gained by atoms of Br to obtain a stable electron configuration? How many valence electrons do the halogens have? A. 1 B. 2 C. 7 D. 8; How many valence electrons do the halogens have? 5, 2, 1 or 7; Write the electron configuration for bromine and state the number of valence electrons. How many protons and electrons .
number of electrons in br|formula to find electrons
PH0 · number of protons in bromine
PH1 · number of neutrons in br
PH2 · number of electrons in boron
PH3 · number of electrons in an atom
PH4 · how to find electron number
PH5 · formula to find electrons
PH6 · electrons in each energy level
PH7 · calculating protons neutrons and electrons
PH8 · Iba pa